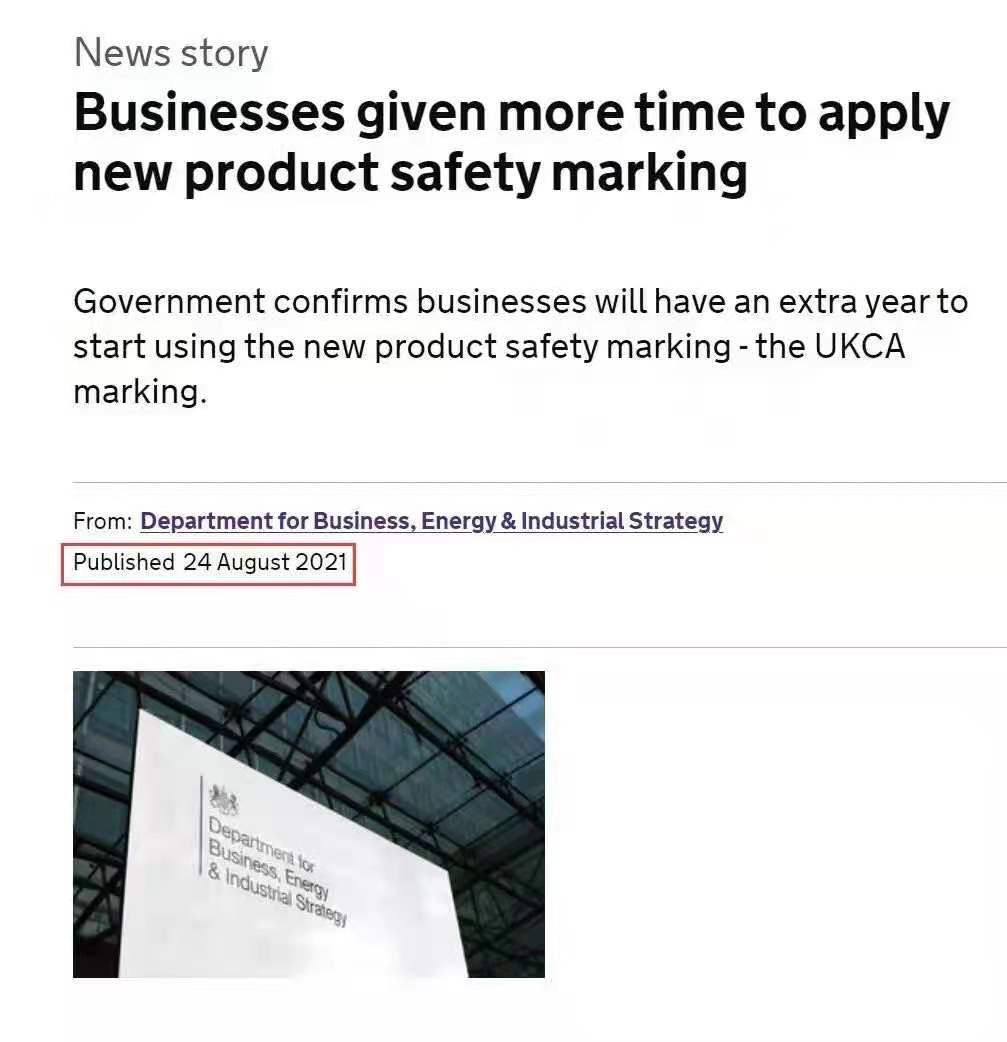
According to the latest guidelines on the use of the UKCA mark issued by the UK official website on August 24, 2021, “Manufacturers can continue to use the CE mark on their products to enter the UK market until January 1, 2023. January 1, 2023 Products on the UK market must be marked with the UKCA logo in accordance with relevant regulations. This means that the acceptance period of the CE mark in the UK market has been extended from January 1, 2022 to January 1, 2023, that is, until January 1, 2023, CE marked products can still be accepted in the UK. But only for products that are consistent with the requirements of the CE mark and the British UKCA mark.

Part of the reason for this extension is: re-testing of products that have always used the CE mark, and the equipment resources for the UKCA mark cannot keep up with the large amount of testing needs. Therefore, the extension of the time to accept the CE mark allows the relevant departments to have more time to establish resource equipment and Perform a retest.
The British authorities encourage manufacturers to ensure full compliance with the requirements of the UKCA mark as soon as possible. From January 1, 2023, the UKCA logo must be used to enter the British Great Britain market. It should be noted that products entering the UK market from the EU area also need a new UK importer to perform the duties of an importer.
This extension means that all products that previously required the CE mark will not need to use the UKCA mark before January 1, 2023. In particular, medical device products do not need to use the UKCA mark before July 1, 2023.
Some screenshots of the official website are as follows:

Warm reminder: The UKCA mark has taken effect. Please start the relevant certification work as soon as possible so that your products can use the UKCA mark in compliance as soon as possible.